Bernard Hanseeuw is a behavioral neurologist conducting research on early Alzheimer’s disease with the aim of better caring for patients with or at-risk for memory decline. He graduated with an MD (2007), PhD (2012) from Université Catholique de Louvain. He then completed a post-doctoral research fellowship in Reisa Sperling’s lab at Harvard Medical School (Boston, USA) from 2014 to 2017. He is now Deputy Head of the Memory Clinic at Saint-Luc University Hospital. He also holds the positions of clinical Associate Professor at UCLouvain and Instructor in Radiology at Harvard University. His research, supported by the National Fund for Scientific Research (FNRS), the Queen Elizabeth Medical Foundation (FMRE), the Belgian Foundation for Alzheimer’s Research (SAO-FRA), the Fondation Saint-Luc and Fondation Louvain, was awarded several prizes including: the ‘Horlait Dapsens’ and ‘Baron Simonart’ Foundations, ‘Prix d’excellence 2018 de la Société Française de Neurologie’ and the ‘Santkin Prize of the Belgian Royal Academy of Medicine’. Dr. Hanseeuw directs the Louvain Aging Brain LAB at the Institute of Neuroscience (https://uclouvain.be/fr/instituts-recherche/ions/neur/the-louvain-aging-brain-lab.html) His motto “increasing knowledge for a better cure” highlights the purpose of his research: guiding clinical trials towards success. His publication list can be found at:
If you like TEDx talks, please enjoy this show:
DETECTING ALZHEIMER’S PATHOLOGY BEFORE THE FIRST MEMORY LOSS
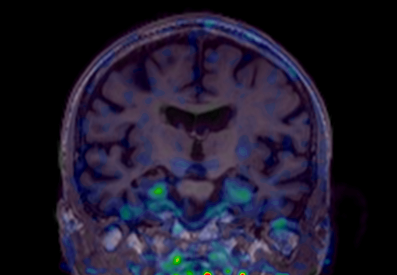
Until a decade ago, a definite diagnosis of Alzheimer’s disease (AD) could only be made after autopsy and confirmation of amyloid and tau pathology in the brain. Neurologists could only give the diagnosis of probable AD to patients with dementia. The absence of biomarkers to confirm the biological diagnosis made it impossible to diagnose AD early, prior to dementia. The Louvain Aging Brain Lab has been among the first in Europe to describe the spatio-temporal progression of Alzheimer’s pathology in living humans, using specific radiotracers and positron emission tomography to detect amyloid and tau deposits in the brain. We have “brought to life” neuropathological classifications, obtained at post-mortem, by transposing them into brain imaging classifications, applicable to detect Alzheimer’s pathology before the onset of symptoms. It appeared that amyloid PET was able to predict which patient would progress to dementia six years later with an overall predictive value of 87%. However, regional amyloid did not predict well the specific cognitive deficits, and up to 20% of cognitively normal older adults do have amyloid pathology in their brain. In contrast, tau PET images (see Figure 1) are much more closely associated to brain dysfunction and cognitive deficits. We are currently developing new ways of assessing subtle cognitive loss, including spatial navigation or linguistic abilities, in apparently normal older adults. This work is of particular importance in the context of upcoming therapeutic trials aiming at preventing the onset of AD in non-demented individuals with evidence of Alzheimer’s pathology. The recent development of blood tests targeting amyloid and tau, of cognitive tests available on smartphone apps, and the large amounts of data collected using brain imaging all require artificial intelligence tool to be analyzed optimally.